Designation process of MDR/IVDR Notified Bodies - update · MDlaw – Information platform on European medical device regulations

Mario Gabrielli Cossellu LinkedIn'de: #notifiedbody #mdr #nando #ivdr #notifiedbodies #conformityassessment…

New Approach Notified and Designated Organisations Information System... | Download Scientific Diagram

Explosives Working Group 4 November 2014 Notified Bodies and NANDO Norma McGovern, DG ENTR.C1 – Internal Market and its International Dimension. - ppt download

Explosives Working Group 4 November 2014 Notified Bodies and NANDO Norma McGovern, DG ENTR.C1 – Internal Market and its International Dimension. - ppt download

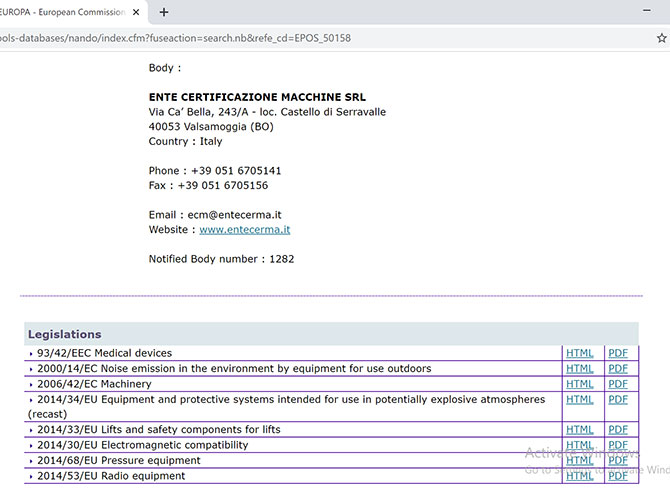
FIND OUT THE ECM ACCREDITATIONS ON NANDO, THE EU DATABASE OF NOTIFIED BODIES - Ente Certificazione Macchine